The question requires us to calculate the number of molecules in 2.65 moles of dinitrogen oxide (N2O).
The Avogadro's number defines the number of units in 1 mol of any substance as 6.022 x 10^23 (units/mol). These units might be atoms, ions, molecules etc. depending on the nature of the substance.
Using the Avogadro's number, we can write:
1 mol N2O -------------------- 6.022 x 10^23 molecules
2.65 mol N2O -------------- x
Solving for x, we'll have:
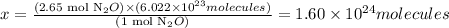
Therefore, there are 1.60 x 10^24 molecules of N2O in 2.65 moles of this substance.