Given,
Mass of the block, m=3.25 kg
Height from which it is dropped, h=75.0 m
The specific heat capacity of the silver, c=234 J/kg/°C
The potential energy stored in the block at a height of 75.0 m is given by,
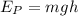
Where g is the acceleration due to gravity.
On substituting all the known values in the above equation,
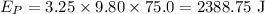
One-third of this energy is converted into heat energy which raises the temperature of the block. Therefore heat supplied is
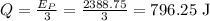
The heat supplied to the block is related to the specific heat and the rise in the temperature as,
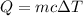
Where ΔT is the rise in the temperature.
Therefore the rise in the temperature is calculated as,
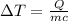
On substituting the known values,
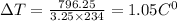
Therefore the rise in the temperature is 1.05 C°
Hence, the correct answer is option (a)