Molarity corresponds to the number of moles of solute in a liter of solution. So, we have the following expression:
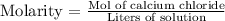
We will use molecular weight (MW) to calculate the moles present in 65 g of calcium chloride.
MW= 110.98 g/mol

So, molarity will be:
Volume: 500mL = 0.5 L
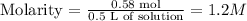
The answer will be 1.2 M