The given molecule C_12 H_22 O_11 is sucrose.
Sucrose of not dissociable in water hence Vant Hoff factor is 1
i.e
i=1
molality=m=0.3
k=freezing point constant (As we don't need to find the perfect freezing point let's keep it constant)

T_f is freezing point
For Sucrose
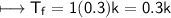
Now.
Let's check Vant Hoff factor of each compound.
AlCl_3=4
CuCl_2=3
NaCl=2
C_6H_12O=1
Let's count Freezing point of each compound.
AlCl_3
m=0.075
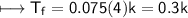
Option A is correct
CuCl_2
m=0.15
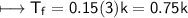
NaCl:-
m=0.3
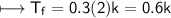
Glucose:-
m=0.6
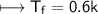
Hence option A is correct
Done