Answer:
6.84L of O2 can be produced.
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:
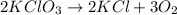
From the balanced reaction we know that 2 moles of KClO3 decompose into 2 moles of KCl and 3 moles of O2.
2nd) It is necessary to calculate the molar mass of KClO3 and O2:
- KClO3 molar mass: 122.6g/mol
- O2 molar mass: 32g/mol
3rd) In the conversion we have to consider:
- KClO3 molar mass.
- The stoichiometry of the reaction between KClO3 and O2: from 2 moles of KClO3, 3 moles of O2 are produced.
- The relation of moles and volume: 1 mole occupies 1 L.
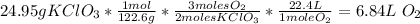
So, 6.84L of O2 can be produced.