To answer this question, we need to:
Step 1 - Balance the question
To balance the equation it is necessary to have the same number of atoms of each element on both sides of the equation (reactant side and product side). Let's count the number of atoms of each element for the unbalanced equation:
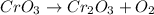
Reactants side:
Cr - 1
O - 3
Products side:
Cr - 2
O - 5
As you can see, chromium and oxygen number of atoms are not equal on both sides of the equation. Let's balance it by changing the stoichiometric coefficient.
First, let's balance chromium, we put coefficient 2 on the reactant side:
2 CrO3 -> Cr2O3 + O2
Now we have 2 chromium and 6 oxygen on the reactant side.
To balance the oxygen, we can put the coefficient 3/2 on the O2
2 CrO3 -> Cr2O3 + 3/2 O2
The equation is balanced, but the coefficients are not whole numbers.
Let's multiply all the coefficients by 2:
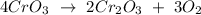
Let's count the number of atoms of each element for the balanced equation.
Reactant side:
Cr - 4
O - 12
Product side:
Cr - 4
O - 12
Now the equation is balanced.
Answer: 4 CrO3 -> 2 Cr2O3 + 3 O2
Coefficients: 4, 2, 3