Answer
655.61 grams of mercury (II) phosphite.
Step-by-step explanation
Given:
Volume, V = 699.9 mL = 0.6999 L
Molarity = 1.233e+00 M
What to find:
The grams of mercury (II) phosphite needed to make 699.9mL of a 1.233e+00 M solution.
Step-by-step solution:
Step 1: Calculate the number of moles of mercury (II) phosphite in 699.9mL of a 1.233e+00 M solution.
The moles can be known using the molarity formula:
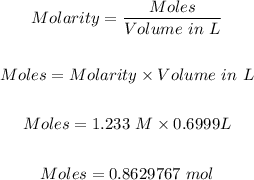
Step 2: Convert the moles of mercury (II) phosphite needed to 699.9mL of a 1.233e+00 M solution to mass in grams.
Using the atomic masses of (Hg = 200.59, P = 30.97, O =15.999) from the periodic table.
The molar mass of mercury (II) phosphite (Hg3(PO3)2) = (3 x 200.59) + 2(30.97 + (3 x 15.999)) = 601.77 + 157.934 = 759.704 g/mol
Therefore, the mass of mercury (II) phosphite needed to 699.9mL of a 1.233e+00 M solution to mass in grams is:
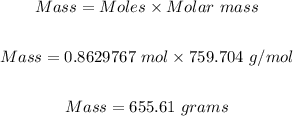
Thus 655.61 grams of mercury (II) phosphite are needed to 699.9mL of a 1.233e+00 M solution.