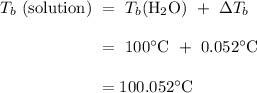Answer:
100.052 degrees C
Step-by-step explanation:
It has been experimentally proven that the addition of solute to water will result in boiling point elevation due to the presence of more molecules. The boiling point elevation refers to the tendency of a solvent's boiling point to increase when an impurity (a solute) is added.
The formula of boiling point elevation is
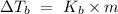 , where m is the molality defined as the number of moles of solute per kilograms of solvent and
, where m is the molality defined as the number of moles of solute per kilograms of solvent and
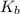 is the molal boiling point elevation constant.
is the molal boiling point elevation constant.
Given that the molal boiling point elevation constant of water is 0.512
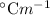 ,
,
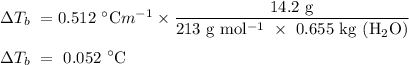
To evaluate the boiling point of the aluminium nitrate solution,