Hello
To calculate the molarmass of the given compound, simply find the individual molar mass of the elements and add them up
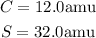
Now, we can calculate the molar mas of the compound
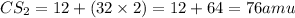
From the calculation above, the molar mass of CS₂ (Carbon Sulfide) is equal to 76amu.