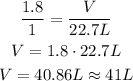STP stands foor standard temperature and pressure, and it is used with the assumintion that the gas behaves as an ideal gas. These conditions are standardized nowadays as temperature of 0°C and pressure of 1 bar.
In this conditions, the volume is directly proportional to the number of moles of the gas.
1 mol in STP conditions occupy 22.7 L.
Using rule of three, we have:
1.8 mol --- V
1 mol --- 22.7 L