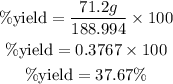Answer:
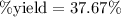
Explanations:
Given the chemical reaction between methane and oxygen expressed as:
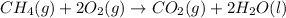
The formula for calculating the percent yield is given as;
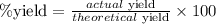
From the given information, actual yield = 71.2 grams
We can determine the theoretical yield from stochiometry.
Determine the mole of Oxygen O2.
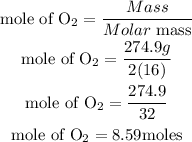
According to stochiometry, 2moles of oxygen (limiting reactant) produce 1 mole of CO2, hence the number of moles of CO2 produced will be given as;
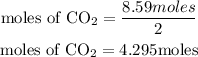
Calculate the theoretical yield (mass of CO2)
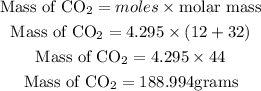
Hence the theoretical yield will be 188.994 grams.
Determine the required percent yield