Let the score in his last game be
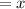
The average of the three games is given as
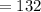
Step 1: The formula for average is
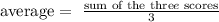
Step 2:Substituting the values of the three scores in the formula above, we will have
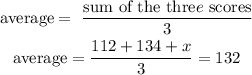
Step 3 : Cross multiply the equation below
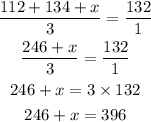
Step 4: Subtract 246 from both sides
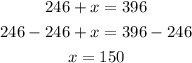
Hence,
John must score a point of 150 to ensure that his average is 132
Therefore,
Final answer = 150