Solution:
Given:
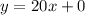
Comparing the equation of the model to the equation of a straight line,

The y-intercept is the y-value when x = 0.
This means when the temperature was 0 degrees celsius, there were no sales of ceiling fan.
Therefore, the number 0 means no sale of fan was made when the temperature was 0 degrees celsius.