Answer:
42. The statement is "The value of ΔH° for the overall reaction is large and negative".
43. The value of the enthalpy change is -93kJ.
Step-by-step explanation:
42. The Kp depends on the ΔH° as both are related by ΔG formula, so the statement that identifies the reason of Kp value is "The value of ΔH° for the overall reaction is large and negative".
If ΔH° is negative, then the calculation of Kp will be a large number, because the formula of Kp is:
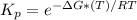
43. To calculate the value of the enthalpy change per mole of HCl produced, it is necessary to write the whole final reaction from the reactions in steps 1, 2 and 3 (multiplying or dividing the enrgies, just like the equations). Then, we have to sum all the final energies:
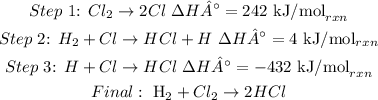
In this case, it is not necessary to multiply or divide the equations, because each H cancels, and each Cl cancels as well.
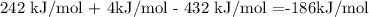
-186 kJ is the enthalpy change for the whole reaction, where 2 moles of HCl are produced. So, to calculate the enthalpy change for 1 mole of HCl, we have to divide it by 2.
Finally, the value of the enthalpy change is -93kJ.