As a solution loses H+ ions its pH increases, i.e. the solution becomes more basic.
In this case, the solution goes from an acidic pH of 6 to a basic pH equal to 10, this means that the concentration of H+ in the solution decreases.
Now, to determine how much is the change of concentration we can apply the following formula:
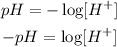
We can clear the H+ concentration by applying the logarithm rules. We have a base 10 logarithm so when clearing we will have:
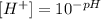
Now we replace the values of each pH and we will have the H+ concentration in each case.
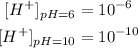
Therefore, the concentration difference will be:
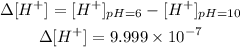
The change of concentration will be 9.999x10^-7