The molarity of a solution is, by definition, the number of moles (n) of solute per liter of solution (V), i.e.:
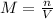
In the first solution, NaOH is the solute. We have 0.6 moles of solute and 1.75 L of solution. We can substitute these values:
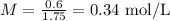
In order to calculate the molarity of the second solution, we must first convert mass (g) to moles, which can be done by using the molar mass of NaCl (58.44 g/mol). We can divide the mass by the molar mass to obtain the number of moles of NaCl:
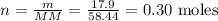
Now we divide this result by the volume of the solution (877 ml or 0.877 L)