The Triangle Inequality Theorem states that any side of a triangle must be shorter than the other two sides added together.
When the three sides are a, b and c, we can write:
![\begin{gathered} aGiven the sides of the triangle 5 cm and 8 cm, we can have that:[tex]\begin{gathered} a=5 \\ b=8 \end{gathered}]()
Therefore, the third side must be less than:
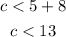
Using the second inequality, we can have:
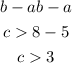
Therefore, the third side must be between the inequality:
[tex]3
Hence, the following options are true:OPTION B
OPTION C
OPTION E