Volume V is inversely proportional to temperature T, when pressure is constant, then:
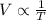
So, for a constant pressure P, we can create the following formula
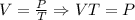
* If the temperature is 50 degrees, the volume is 20 cubic feet. What will the volume be when the temperature is 100 degrees.
Since P is mantain constant, then
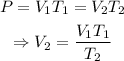
Where, the variables are:
V1 is the initial volume = 20 cubic feet
T1 is the initial temperatura = 50 degrees
V2 is the final volume in cubic feet
T2 is the final temperature = 100 degrees
Now, let's replace into the equation
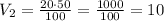
Thus, the solution is 10 cubic feet