Percent error is used to calculate the difference between the true value versus the value found experimentally. It is calculated with the following equation:
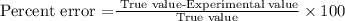
Experimental value =0.994g/cm3
True value = 0.9973 g/cm3
Now, we will replace the known values in the equation:

So, the percent error will be 0.33%