INFORMATION:
We have:
- 66 grams of NaCl
- 255 grams of water
If the NaCl is dissolved into the water, we must find the mass percent
STEP BY STEP EXPLANATION:
To find the mass percent we must must use the next formula
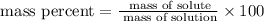
In our case,
- the solute is NaCl
- the solution would be the sum of masses from NaCl and water
Then,
- mass of solute = 66 g
- mass of solution = 66 g + 255 g = 321 g
Finally, replacing in the formula,
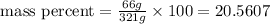
ANSWER:
The mass percent when 66 grams of NaCl is dissolved in 255 grams of water is 20.60%