Answer
50.6 kJ
Step-by-step explanation
According to the given equation:
2NaHCO3 (s) + 85 kJ --> Na2CO3 (s) + H2O (I) + CO2 (g)
85 kJ of heat decomposed 2 moles of NaHCO3
1 mole of NaHCO3 = 84.007 g/mol
2 moles of NaHCO3 = (2 x 84.007) = 168.014 grams
So if 168.014 grams NaHCO3 required 85 kJ
100.0 grams NaHCO3 will require
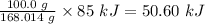
Therefore, the heat required to decompose 100.0 grams of NaHCO3 is 50.6 kJ