Answer:
The molarity of the solution is 0.19M. (first option)
Step-by-step explanation:
The given information from the exercise is:
- Moles of NaCl (n): 0.65 moles
- Volume of solution (V): 3.50L
To calculate the molarity of the solution we have to replace the values of n and V in the Molarity formula:
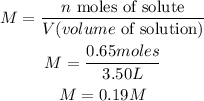
So, the molarity of the solution is 0.19M.