Answer:
32.99g of H3PO4 are formed.
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:
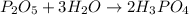
From the balanced reaction we know that 1 mole of diphosphorus pentoxide (P2O5) reacts with 3 moles of water (H2O) to produce 2 moles of phosphoric acid (H3PO4).
2nd) Using the molar mass of P2O5 (142g/mol), H2O (18g/mol) and H3PO4 (98g/mol) we can convert moles into grams:
• P2O5: in this case there is only 1 mole so it is equal to 142g.
,
• H2O:
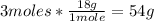
• H3PO4:
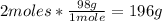
Now we know from the balanced reaction that 142g of P2O5 react with 54g of H2O to ptroduce 196g of H3PO4.
3rd) It is necessary to determine which reactant is the limiting reactant and which reactant is the excess reactant.
So, we have to use the information of the balanced reaction and the given values of P2O5 (23.9g) and H2O (9.1g):
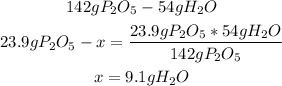
In this case, we have the exact amount of each reactant to produce H3PO4 without leftover reactants.
4th) We can calculate the grams of H3PO4 that will be produced using the given value of P2O5 (or H2O):
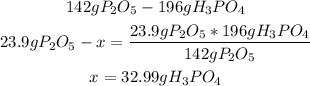
Finally, 32.99g of H3PO4 are formed.