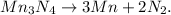Answer:
![1Mn_3N_4\operatorname{\rightarrow}3Mn+2N_2.]()
Step-by-step explanation:
We can balance the chemical equation by doing the trial and error method.
You can see that we have 3 Mn on the reactants, and 1 Mn on the products. And, we have 4 N on the reactants, and 2 N on the products.
If we put '3' moles beside Mn and '2' moles beside N4, we will obtain the balanced chemical equation, because we're going to have 3 moles of Mn on both sides, and 4 moles of N on both sides too: