Step 1 - Understanding the definition of molar concentration
Molar concentration (M or mol/L) is defined as the quotient between the number of moles (n) and the total volume of the solution (V):
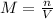
Therefore, if the volume of the solution and its final concentration are known, we can use the equation to find the required number of moles.
Step 2 - Finding the required number of moles
The solution's concentration is 0.46 M and its volume is 250 ml. The volume must always be used in L, so:
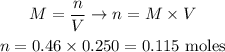
Therefore, 0.115 moles would be required.