Answer: 4.123 x 10^22
Step-by-step explanation:
The first step is to determine how many moles of sulfur hexafluoride (SF6) there are. To do this, you must take the mass of the SF6 (10g) and divide it by the molar mass (146.06).
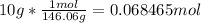
Then you must use Avagadros number to find the number of molecules.
Avagadros number is the number of molecules in 1 mol of a substance, so a simple multiplication will do the trick.
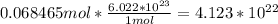
And that is your answer. There are 4.123 x 10^22 molecules of sulfur hexafluoride in 10g of it.