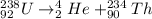Answer:
Uranium-238
Explanations:
The process by which an atomic nucleus looses energy by radiation is known as radioactivity.
An alpha particle, have an atomic number of 2 and a mass number of 4 as shown;
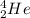
When this is combined with Th-234, it forms Uranium-238. This shows that the uranium nucleus emits alpha particle to form Th-234 according to the chemical equation below;