The question requires us to chose among gases A, B, C and D which one would present the smallest volume, given the number of moles, pressure and temperature of each gas.
The ideal gas equation relates the pressure, volume, number of moles and temperature of an ideal gas as it follows:
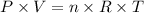
where P is the pressure of the gas, V is its volume, n corresponds to the number of moles of gas, T to the temperature and R is the constant of gases.
We can rearrange the equation above to look specifically at the volume of the gas:
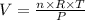
We can note from the equation above that the volume of a gas should increase with its number of moles and temperature (directly proportional), and decrease with the increase of pressure (inversely proportional). Based on this, we could infer that the gas with smallest pressure would present the biggest volume - but, as the question provided values of n and T and they are not the same for all gases, it is not a completely correct affirmation in this case.
Since the question provided the values of n, P and T for all samples, we can calculate the volume of each gas using the equation above and determine which one would present the smallest volume. Not that, as R is a constant and won't change, we don't need to add it to the calculation (we'll keep it as the variable R and it won't affect the values if they are only considered to compare the volume of gases):
![\begin{gathered} V_A=\frac{(2\text{mol)}*(273K)* R}{\mleft(760\operatorname{mm}\mright)}=0.718R \\ \\ V_B=\frac{(1\text{mol)}*(273K)* R}{(380\operatorname{mm})}=0.718R \\ \\ V_C=\frac{(1\text{mol)}*(273K)* R}{(760\operatorname{mm})}=0.359R \\ \\ V_D=\frac{(2\text{mol)}*(546K)* R}{(760\operatorname{mm})}=1.437R \end{gathered}]()
Therefore, considering the information given, the gas with smallest volume would be gas C and the best option to answer the question is letter C.