The balanced reaction of the hydrogen sulfide decomposition reaction is as follows:
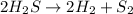
We see that for every two moles of H2, one mole of S2 is formed. Now, let's determine how many moles of H2 are formed. For that we divide the mass they give us by the molar mass of H2. The molar mass of H2 is: 2.016 g/mol
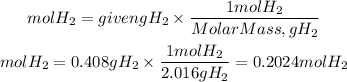
The S2 to H2 ratio is 1/2. Therefore the moles of S2 obtained will be:
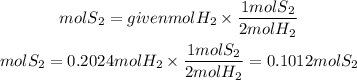
We multiply the moles found by the molar mass of S2. The molar mass of S2 is: 64.13g/mol
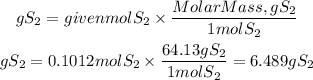
Answer: It will be obtained 6.489 g of sulfur