ANSWER
The number of moles of argon in 354g of argon is 8.615 moles
Explanation:
Given parameters
• Mass of argon = 354g
,
• The molar mass of argon = 39.948g/mol
To find the number of moles, we need to apply the mole formula
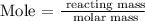
The next thing is to substitute the above parameters into the formula
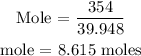
Therefore, the number of moles of argon in 354g of argon is 8.615 moles