To answer first we need to calculate the molar weight of this molecule (KBr):
For this we go to the periodic table and check the molar weight of potassium and bromine:
K: 39.098 g/mol
Br: 79.904 g/mol
So the molar weight of KBr is:
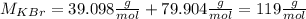
Now, we know that the solution is 0.955 M, this means that in 1000 ml there are 0.955 moles of KBr. So we calculate the number of moles in 92.7ml:
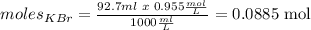
Now we use the molar weught to calculate the grams in the sample:
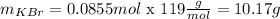
So the answer is 10.17g.