
Given :-
- We have given equation Cacl2 + K2CO3
Let's Begin
We have ,
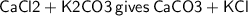
Let's balance it
On reactant side,
On product side,
Therefore, Balanced equation will be :-
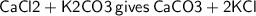
- The above reaction is Double displacement reaction.
Double Displacement reaction :-
- Double displacement reaction is a reaction in which the ions of compound on a reactant side exchanges due to the varying reactivity of elements so that they can form new products.