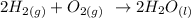Answer:
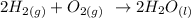
Step-by-step explanation:
Here, we want to get the equation of reaction between hydrogen and oxygen
When hydrogen and oxygen react with the ration 2 to 1 in terms of number of moles, water is produced (2 moles of water)
We can show that as follows;