Answer
The pressure change = 3.11 atm
Step-by-step explanation
Given:
Initial pressure, P₁ = 3.00 atm
Initial temperature, T₁ = 25.0 °C = (25.0 + 273.15 K) = 298.15 K
Final temperature, T₂ = 36.0 °C = (36.0 + 273.15 K) = 309.15 K
What to find:
The final pressure, P₂.
Step-by-step solution:
The final pressure, P₂ of the gas can be calculated using the Amonton's law equation/formula given below:
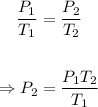
Putting the values of the parameters into the formula, we have:
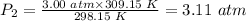
Therefore, the pressure change when a constant volume of gas at
3.00 atm is heated from 25.0 °C to 36.0 °C = 3.11 atm.