The equation presented to us is not balanced. We must balance it first. The elements that are not balanced are chlorine and hydrogen. We have two atoms of those elements in the reactants, so we put the coefficient two in front of the HCl molecule to balance.
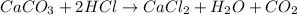
Now, the reaction is balanced, since we have the same number of atoms of each element on each side of the reaction. Now we must calculate the limiting reagent since it will be based on this reagent that we will do the calculations to find the mass of CaCl2.
To find the limiting reactant we calculate the moles of each reactant using its molar mass.
Moles of CaCO3
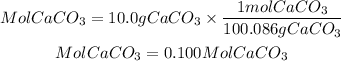
Moles of HCl
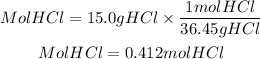
By stoichiometry, we can calculate the ratio HCl to CaCO3. We need two moles of HCl for one mole of CaCO3 to react. So the ratio HCl to CaCO3 is equal to 2/1. That is the theoretical ratio. The current is found by dividing the moles of HCl by those of CaCO3.
The actual ratio HCl to CaCO3 is 0.412/0.1=4.1
The current ratio is higher than the theoretical one, which means we have enough HCl to react. This will be the excess reagent. So the limiting reactant will be CaCO3.
By stoichiometry, we have that 1 mol of CaCO3 produces 1 mol of CaCl2. So, the maximum moles produced will be the same moles of CaCO3 available. This is 0.100mol of CaCl2.
The mass of CaCl2 will be:
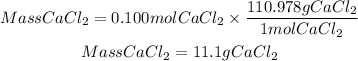
The maximum mass of calcium chloride (CaCI2) that can form is 11.1 g of CaCl2.