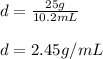Answer:
2.45 g/mL
Step-by-step explanation:
(assuming that the original volume was 20 mL and not L)
Since you know the amount of water in the cylinder before and after you placed the rock in, you can subtract them to get the volume of the rock itself.
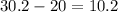 mL.
mL.
To get the rock's density, use the equation
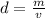 , where m is mass and v is volume.
, where m is mass and v is volume.