We want to consume 0.0424 moles of sucrose. We need to find the grams using the molar mass of sucrose which is 342 g/mol.
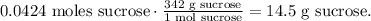
With this data, we can do a rule of three to find the amount of cereal that contains 14.5 gr of sucrose, like this:
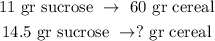
And the calculation would be:
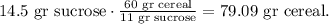
The answer is that we're going to eat 79 grams of cereal if we want to consume 0.0424 moles of sucrose.