Explanations:
According to the pressure law, the pressure (P) of a fixed mass of a gas is directly proportional to its absolute temperature (T) provided that the volume remains constant. Mathematically;
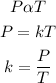
k is the variation constant
If we have initial and final pressure and initial and final temperature, the formula can be written as:
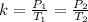
3) The gas law that demonstrates the equation and definition above is known as the PRESSURE LAW.