Step 1 - Finding an equation to solve the problem
For a gas, temperature, volume and pressura are importante variables. They are related with the number of moles of gas by the following equation:
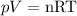
In this equation, R is the universal gas constant, which is 0.082 L.atm/mol.K
Step 2 - Substituting the values on the equation
From the exercise, we know that:
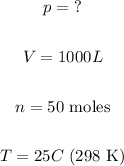
Substituting these values on the equation, we obtain thus:
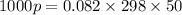
Working on the math, we have:
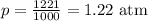
The pressure will be thus 1.22 atm.