To solve this question, we would use mole concept
step one
write a balanced chemical equation
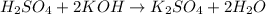
Step two
Using the statement given,
0.55 mol of H₂SO₄ = 1000mL
x mol = 12.0mL

Going back to the balanced chemical equation,
1 mole of H₂SO₄ reacted with 2 moles of KOH
This implies that 0.0066 moles of H₂SO₄ will react to y
Cross multiply both sides and solve for y.
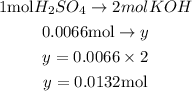
Now we can finally proceed to find the volume of KOH used.
If 0.739moles is present in 1000mL of KOH
0.0132 moles is present in z mL
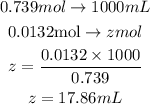
From the calculation above, 17.86mL of KOh reacted with 12.0mL of H₂SO₄