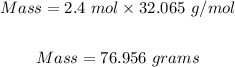Answer
76.965 grams
Step-by-step explanation
Given:
2.4 moles sulfur.
What to find:
To convert 2.4 moles of sulfur to grams.
Solution:
The given moles of sulfur can be converted to grams using the mole formula below:
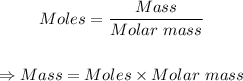
From the periodic table, the molar mass of sulfur = 32.065 g/mol.
Plug in moles = 2.4 mol, and molar mass = 32.065 g/mol into the formula: