First, we have to convert from 195 grams of KClO3 to moles, using its molar mass which you can calculate it using the periodic table (122.5 g/mol):
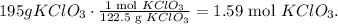
Now that we have this value, we can find the number of moles produced of oxygen doing a rule of three taking into account that 2 moles of KClO3 produces 3 moles of oxygen:
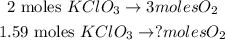
And the calculation would be:
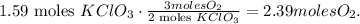
The final step is to convert 2.39 moles of oxygen to grams using its molar mass. The molar mass of oxygen (O2) is 32 g/mol:
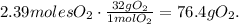
The answer is that 195 grams of KClO3 decomposed, produces 76.4 grams of oxygen (O2).