Answer:
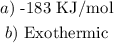
Step-by-step explanation:
Here, we want to calculate the total bond energy and state if the reaction is exothermic or endothermic
a) Calculation of bond energy
Mathematically, that would be the difference between the energy of the bond formed (products) and the energy of bonds broken (reactants)
2 H-Cl bonds formed (energy out)
1 H-H bond in
1 Cl-Cl bond in
We have the bond energy as:
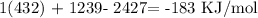
b) The reaction is exothermic because the total change in bond energy is negative. This is an indication of the fact that energy is released as the supplied energy (energy from reactants) is greater than the output energy (energy of products).