Answer:

Step-by-step explanation:
We are asked to find how much energy is required to heat a sample of water.
We will use the following equation:
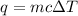
The mass of the water is 32.5 grams. The specific heat capacity of water is 4.18 Joules per gram degree Celsius.
The change in temperature is the difference between the final temperature and the initial temperature. The water's temperature is raised from 34 degrees Celsius to 75 degrees Celsius.
- ΔT= final temp - inital temp
- ΔT = 75 °C - 34 °C = 41 °C
Now we know all three variables:
- m= 32.5 g
- c= 4.18 J/g °C
- ΔT = 41 °C
Substitute the values into the formula.
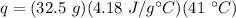
Multiply the first 2 numbers together. The units of grams cancel.
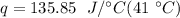
Multiply again. This time, the units of degrees Celsius cancel.
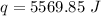
5,569.85 Joules of energy are required.