Answer: There are 27.3 grams of Na2SO4 in 0.192 moles of this compound
Step-by-step explanation:
The question requires us to calculate the amount of sodium sulfate, Na2SO4, that are in 0.192 moles of this compound.
The number of moles of a substance can be calculated from the mass of the sample, in grams, and the molar mass of the substance, in grams per mol:
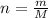
where n is the number of moles, m is the mass and M is the molar mass.
If we rearrange the equation above, we can calculate the mass of a sample from the number of moles and molar mass of a compound:
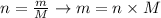
Thus, in order to calculate the mass of Na2SO4 in 0.192 moles of this compound, we need to determine the molar mass of Na2SO4.
The atomic masses of Na, S and O are 22.99, 32.06 and 15.99 amu. Then we can calculate the molar mass of Na2SO4 as:
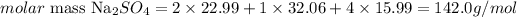
Now that we have the molar mass of Na2SO4 (142.0 g/mol) and the number of moles in the sample (0.192 mol), we can determine the mass of the sample using the previous equation:
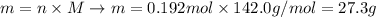
Therefore, there are 27.3 grams of Na2SO4 in 0.192 moles of this compound.