According to the Brønsted-Lowry Definition of Acids and Bases, an acid is a substance that after the reaction has a lower concentration of H+ ions, while the base accepts these H+ ions and will have a higher concentration. Let's see what is the reaction and the reaction mechanism:
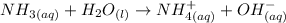
We see that the water donates the H+ ion, therefore the water will be the acid.
Ammonia accepts the ion H+, therefore ammonia will be the base.
Answer:
Water --->Acid
Ammonia --->Base