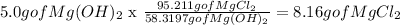According to the Chemical equation:
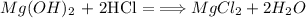
Which means that for one mol of Mg(OH)2 consumed, one mol MgCl2 is produced. when we change mols to mass ussing the molar mass of the components this means that for every 58.3197 g of Mg(OH)2 consumed 95.211 g of MgCl2 is produced. But instead of 58.3197 grams we start with 5.0 g of Mg(OH)2. then we need to make the following calculation: