Answer
A. Acidic
Step-by-step explanation
Given:
[H⁺] = 2.65 x 10⁻² M
Note that: the pH values range from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base.
Also, the pH of a solution can be determine using the pH formula below:
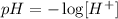
So pH = - log [2.65 x 10⁻²]
pH = 1.58
The pH of the solution fall in the acidic range. Hence, the solution acidic.