the The concentration of a solute is defined as the quantity of solute per unit quantity of solution,
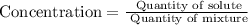
Given that the initial volume is 80 mL and the corresponding concentration is 3.5%. So the quantity (x) of solute can be obtained as,
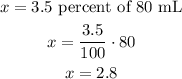
This solution is mixed with pure water, so the quantity of solute in the final mixture remains same since no solute is added.
The final volume of the mixture is 160 mL.
So we know the quantity of solute and the quantity of solution. Therefore, the concentration C of the final solution is calculated as
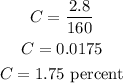
Thus, the concentration of the final solution is 1.75%.