Answer
Energy required to melt ice (Q) = 83500 j
Step-by-step explanation
Given
Mass of ice = 250 g
Required: Energy to melt ice
Solution
Ice melts at B, where the temperature is 0 °C.
To solve this question, we will use:
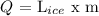
where Q is the amount of heat needed to melt the ice, L ice is the Latent Heat (or heat of fusion of water = 334 j/g) of melting for ice, and m is the given mass of ice.
Q = 334 j/g x 250 g
Q = 83500 j